Patients Breathe Easier with New Generic Advair Option
Client: Hikma Pharmaceuticals
The Challenge:
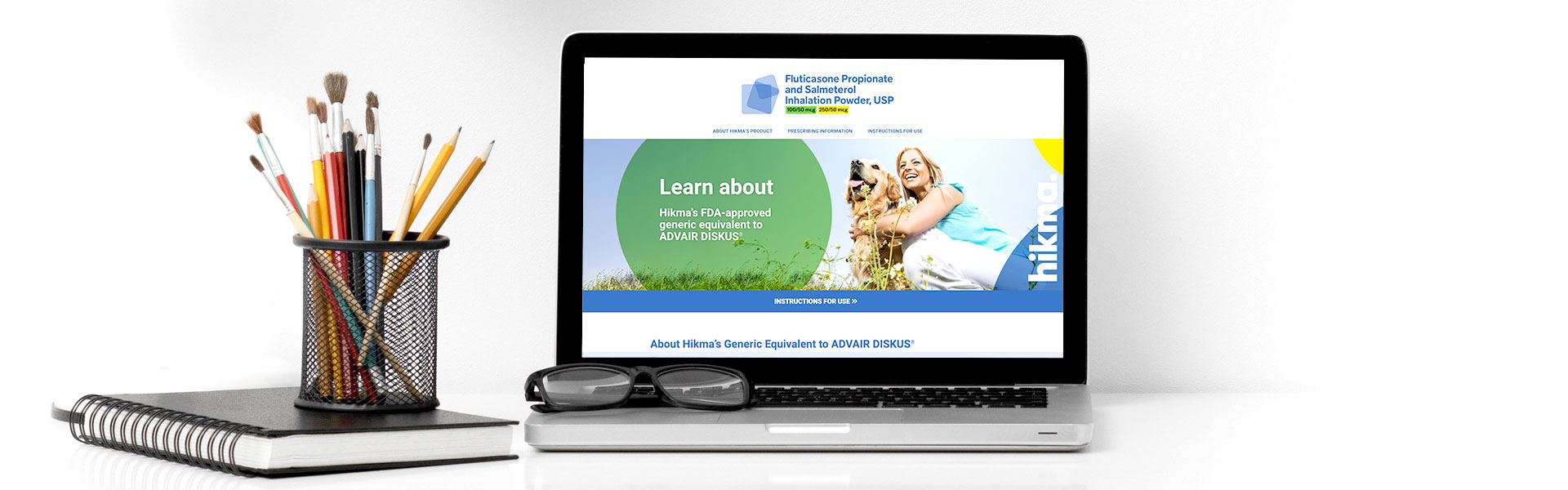
With the thumbs up from the FDA, Hikma Pharmaceuticals partnered with Responsory to launch its generic version of GlaxoSmithKline’s Advair Diskus®. Working with the product team, Responsory used a proprietary AI-powered platform to gather consumer intel. This included identifying what audiences use this medication, and their unique questions, objections and perceptions about the drug.
Client: Hikma Pharmaceuticals
The Solution:
With a solid understanding of the product, market and audience, one of our priorities was to increase awareness of the active ingredients in Advair Diskus® and Hikma’s generic Fluticasone Propionate and Salmeterol Inhalation Powder, USP are the same. The noticeable difference for patients is the inhaler device. Hikma worked with Vectura Group to develop the proprietary dry powder inhaler and formulation technology.
Responsory’s pharmaceutical team then worked briskly behind the scenes to help the multinational company create a brand identity, collateral and website where patients would find product information along with an “Instructions for Use” guide to educate patients on how to effectively administer the medication.
The Result:
Leveraging AI insights for effective branding and patient engagement, our collaboration highlighted therapeutic equivalence and inhaler device innovation. Demonstrating our understanding of the generics market and commitment to enhancing patient experiences, this successful product launch paved the way for future collaborations with Hikma.